Why the long leadtime?
It’s the bane of our lives, the one thing that holds us back from starting the next campaign and there’s not a lot we can do about it. Or is there?
The uptake of single use systems assemblies has increased dramatically year-on-year in the last decade. This is mainly due to the flexibility SU systems bring to manufacturing for multi-product facilities and low capital costs for new manufacturing processes.
For many suppliers, the introduction of standardized SU product ranges has largely addressed the lead time issue in the industry. However, the vast majority of SU end-users still prefer to have completely customized single-use solutions. It’s called the 80-20 rule. 80% of the single-use assemblies sold are customized and 20% are the standard off-the-shelf product.
This results in a complex supply chain where only the standard components such as bag chambers, sanitary connections, quick connect couplers, Luer slips, air vents and tubing such as platinum cured silicone; are kept in stock, ready for assembly. Anything a little less commonly used (e.g. specific brands of media filters, hollow fibres, and proprietary connectors), is not generally kept in stock and can often end up being the bottleneck in the assembly process.
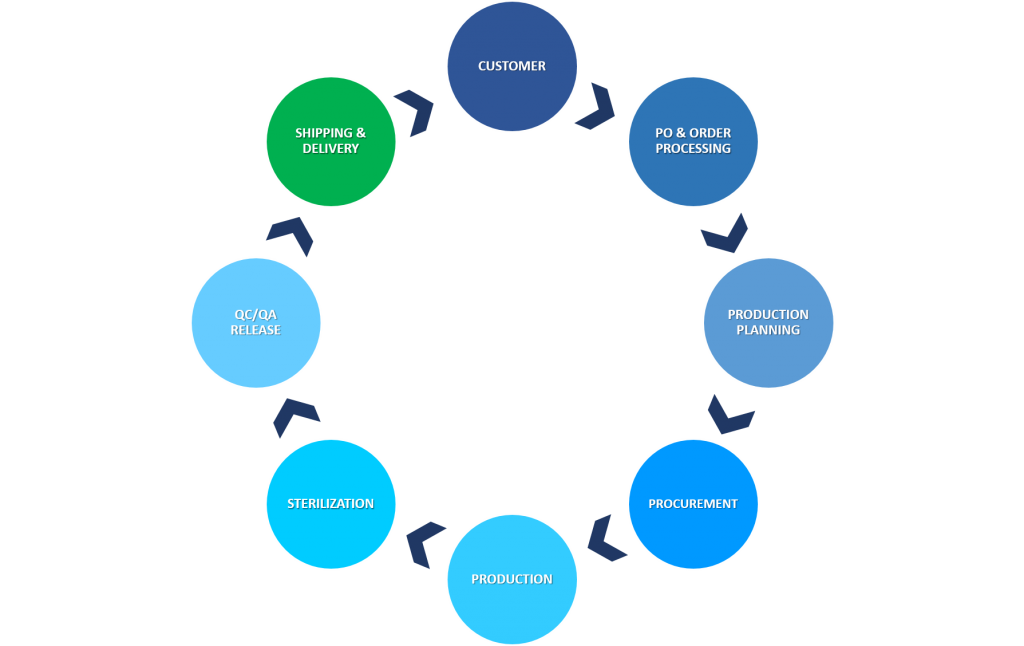
Common Bottle Necks in Custom Single Use Assembly Manufacture:
- Procurement and Qualification of components: Many components used in single-use assemblies have long lead times due to the manufacturing process or raw material availability. The lead time of components ranges from 2 days to 10 weeks, and in some cases can be as long as 24 weeks.
- Production: This part of the assembly process normally takes 1-2 weeks, however, bottlenecks can occur due to capacity issues, or even during one-off events such as pandemics where priority is often given to customers who are directly involved in the supply of vaccines and therapies for that event.
- Sterilization: Reduced capacity of the irradiation suppliers due to increasing demand. Therefore, the gamma irradiation process typically takes 1 week for completion.
- QA/QC Release: Once the product returns from the sterilization plant, a full quality release takes approx. 2-3 days, but can take longer if higher levels of quality testing have been requested.
- Shipping & Delivery: 3-10 days depending on where the assembly has been manufactured.
For customized assemblies, therefore, the best case scenario with most suppliers is a 4-week lead time, but a 12 – 16 week lead time is much more common now due to current demand in the industry.
Figure 1: From purchase order to delivery: the steps involved in producing customized single use assemblies
Supply Chain Risk Mitigation Strategy
If the most variable parts of the process involve components from 3rd party suppliers, then what is the best course of action when ordering customized assemblies from a single-use supplier?
There are a few risk mitigation strategies that can be considered:
- Validate a 2nd Supplier / Dual Supply. This is where the customer validates 2 suppliers for the same assemblies, minimizing the risk in their supply chain. This can be carried out by each supplier using exactly the same component in the assembly or also by having “like-for-like” components (for example one supplier using C-flex and the other A-Flex).
- Warehousing & Call-off. While most manufacturers try to stick to just-in-time ordering, there is a lot to be said for forecasting with your suppliers and stocking up on heavily used assemblies on your behalf. Call-Off orders are an effective way to manage end-user usage to eliminate long lead times and the risk of stockouts.
- Use a Local Supplier. Very often, global SU suppliers concentrate on high-volume assemblies and customers, meaning that customers with small-batch needs can wait for prolonged periods to receive their orders. Using a local supplier can bring many benefits, from quick turn-around to the ability to work with any customer-specified components.
Single-use assemblies were designed for flexibility: to allow manufacturers to respond quickly to emerging diseases, new therapies, and customer demand.
Isn’t it time you worked with a SU supplier that is as flexible as you?
Contact ESI Ultrapure today via Chat or leave us a message on our enquiry form, we will be happy to discuss your requirement.

